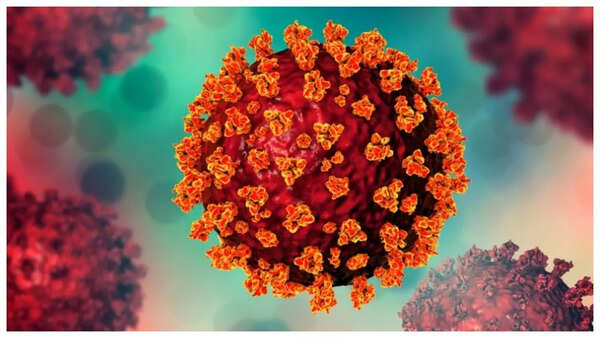
Dengue fever, a viral infection transmitted by the Aedes aegypti mosquito, poses a significant health challenge, particularly in tropical regions. The infection can manifest in varying degrees of severity, from mild flu-like symptoms to severe complications, including fatalities, especially in individuals with weakened immune systems.
India accounts for a substantial portion of the estimated 100–400 million dengue infections worldwide. The incidence of the disease typically peaks during the monsoon season. However, there is now a beacon of hope on the horizon. The country's first dengue vaccine is in the advanced stages of testing. Phase 3 trial enrollment is nearing completion.

Dengue fever, transmitted by Aedes mosquitoes, triggers a range of debilitating symptoms. These include high fever, severe headaches, intense joint and muscle pain, and a potentially dangerous drop in blood platelet count. In severe instances, dengue can lead to hemorrhaging, organ failure, and even death. Children and the elderly are particularly vulnerable to the most severe forms of the disease.
The development of a safe and effective dengue vaccine has been a long-standing challenge for scientists. One of the major hurdles is the existence of four distinct serotypes of the dengue virus. An effective vaccine must provide protection against all four. While some vaccines have been developed in other countries, they have demonstrated inconsistent results and have not been widely adopted.
The Serum Institute of India (SII) is developing India’s first dengue vaccine. TetraVax-DV, as the vaccine is called, is engineered to confer protection against all four dengue virus serotypes. The vaccine employs a weakened form of the virus. This will stimulate the body’s immune system to develop defenses against dengue. Importantly, because it does not contain a live virus, it cannot cause the disease itself.
Key features of the vaccine:
Before a vaccine can be considered for widespread use, it must undergo a series of rigorous testing phases:
Phase 3 is a critical stage. It demonstrates whether the vaccine can effectively prevent dengue transmission within the community.
The Phase 3 trial for India’s dengue vaccine commenced in 2023. The trial spans more than 20 sites across India, encompassing both urban and rural settings. The trial aims to enroll over 10,000 volunteers. These volunteers will include children and adults from diverse backgrounds. Enrollment is nearly complete, and preliminary data indicates promising results.
Monitoring: Volunteers will be closely monitored for any potential side effects and to assess the duration and strength of their protection against dengue.
Data Analysis: Scientists will meticulously analyze the data collected during the trial to determine the vaccine's overall efficacy and safety profile.
Approval: If the trial results are favorable, Indian health authorities could grant approval for the vaccine within the next year.
India bears a disproportionately high burden of dengue cases globally. Seasonal outbreaks strain healthcare resources and cause significant hardship for affected families. A safe and effective vaccine has the potential to:
Regardless of the availability of a vaccine, preventive measures remain crucial in mitigating the risk of dengue. These include employing mosquito repellents, wearing protective clothing, eliminating standing water sources to prevent mosquito breeding, and ensuring proper drainage systems.
Source: The Indian Council of Medical Research (ICMR) and Panacea Biotec.
Newer articles
Older articles
 Vitamin D Could Slash Tooth Decay Risk by 50%, Study Suggests
Vitamin D Could Slash Tooth Decay Risk by 50%, Study Suggests
 Indian Cricket Star Mukesh Kumar and Wife Divya Singh Announce Birth of Son
Indian Cricket Star Mukesh Kumar and Wife Divya Singh Announce Birth of Son
 Shubman Gill's Captaincy Under Fire: Bold Calls Needed After England Test Defeat
Shubman Gill's Captaincy Under Fire: Bold Calls Needed After England Test Defeat
 Microsoft Aims for Foldable Redemption with Novel Hinge Design to Rival iPhone and Android
Microsoft Aims for Foldable Redemption with Novel Hinge Design to Rival iPhone and Android
 Popular Finance YouTuber's Account Hacked, Bitcoin Scam Promoted: Security Lessons Learned
Popular Finance YouTuber's Account Hacked, Bitcoin Scam Promoted: Security Lessons Learned
 Esha Gupta Breaks Silence on Hardik Pandya Romance Rumors: 'We Were Just Talking'
Esha Gupta Breaks Silence on Hardik Pandya Romance Rumors: 'We Were Just Talking'
 Hollywood's Love Affair with India: Iconic Film Locations Revealed
Hollywood's Love Affair with India: Iconic Film Locations Revealed
 Rishabh Pant Aims to Surpass Virat Kohli in Test Century Tally During England Series
Rishabh Pant Aims to Surpass Virat Kohli in Test Century Tally During England Series
 Prithvi Shaw Credits Sachin Tendulkar's Guidance for Career Revival After Setbacks
Prithvi Shaw Credits Sachin Tendulkar's Guidance for Career Revival After Setbacks
 Ashada Gupt Navratri 2025: Unveiling Dates, Timings, Significance & Secret Rituals
Ashada Gupt Navratri 2025: Unveiling Dates, Timings, Significance & Secret Rituals